Fourteen billion years ago, when the hot, dense speck that was our universe quickly expanded, all of the matter and antimatter that existed should have annihilated and left us nothing but energy. And yet, a small amount of matter survived.
We ended up with a world filled with particles. And not just any particles—particles whose masses and charges were just precise enough to allow human life. Here are a few facts about the particle physics of you that will get your electrons jumping.
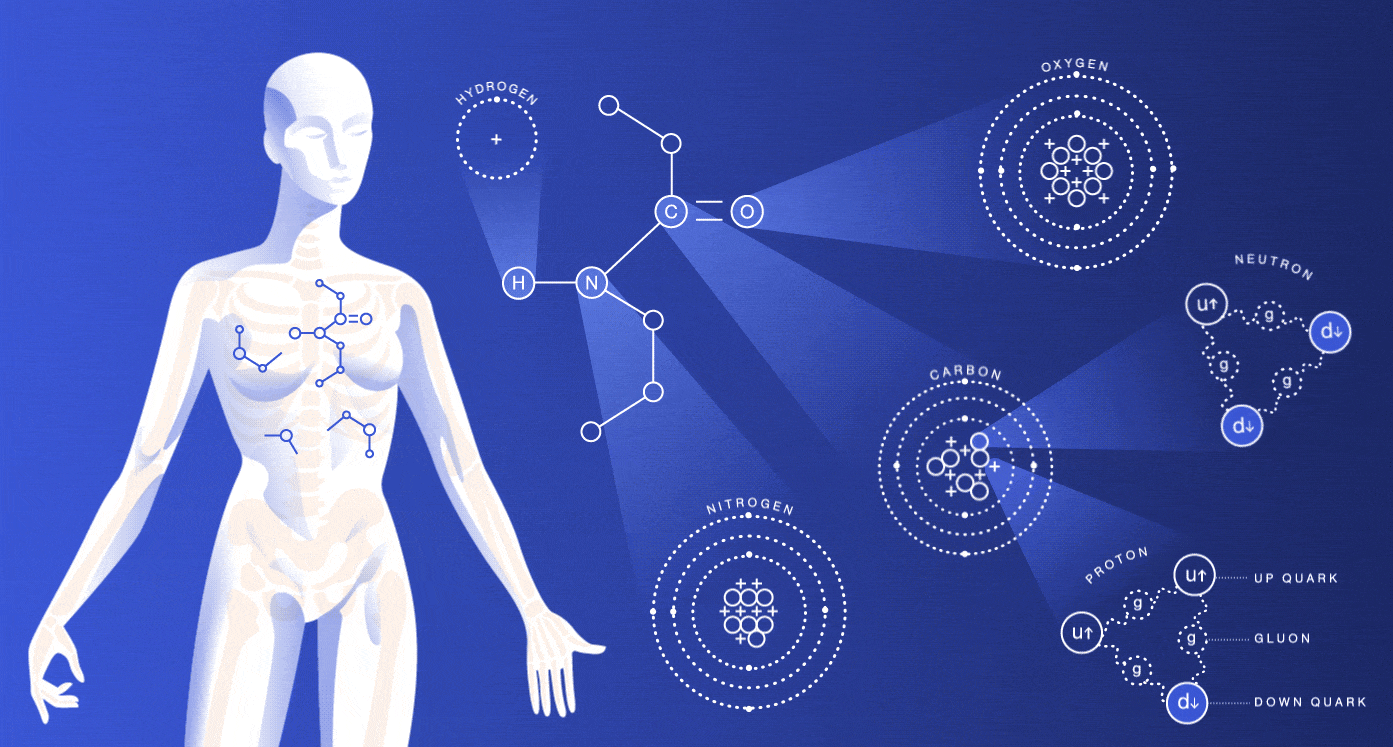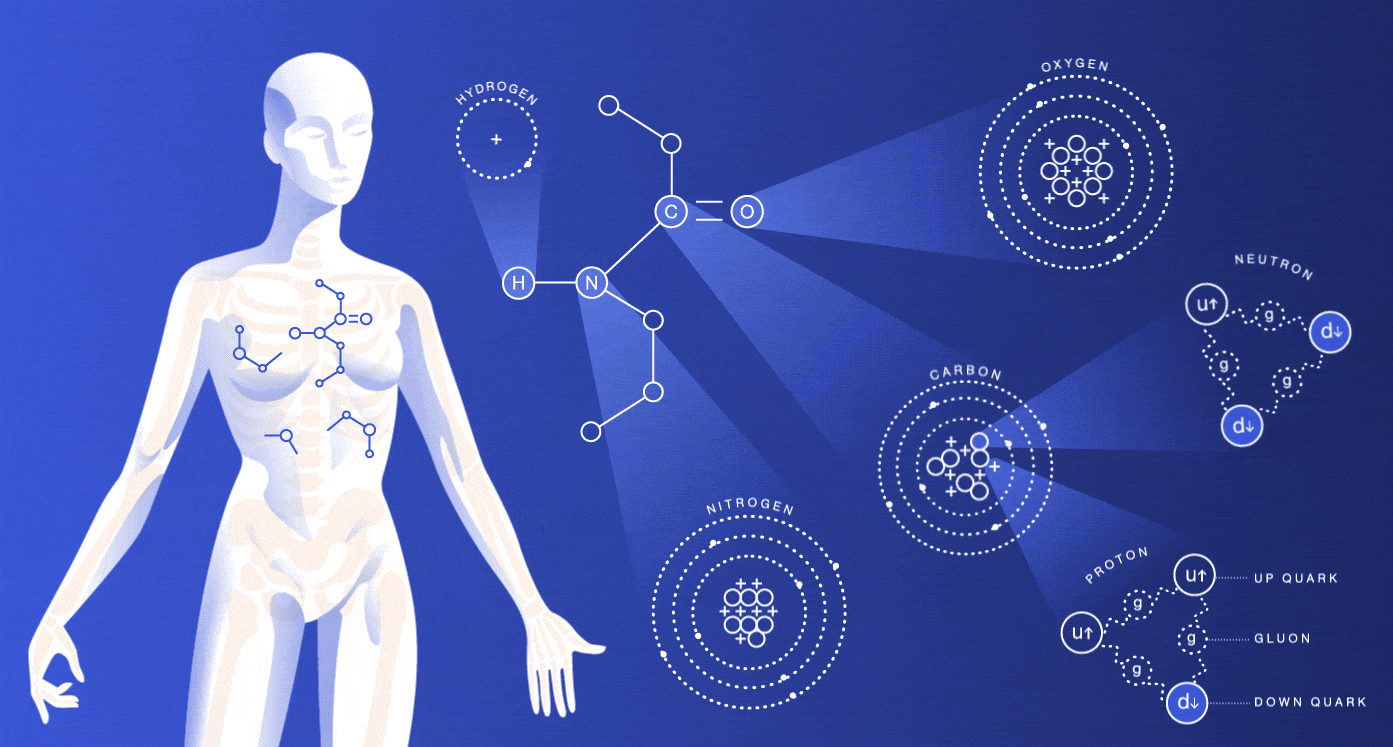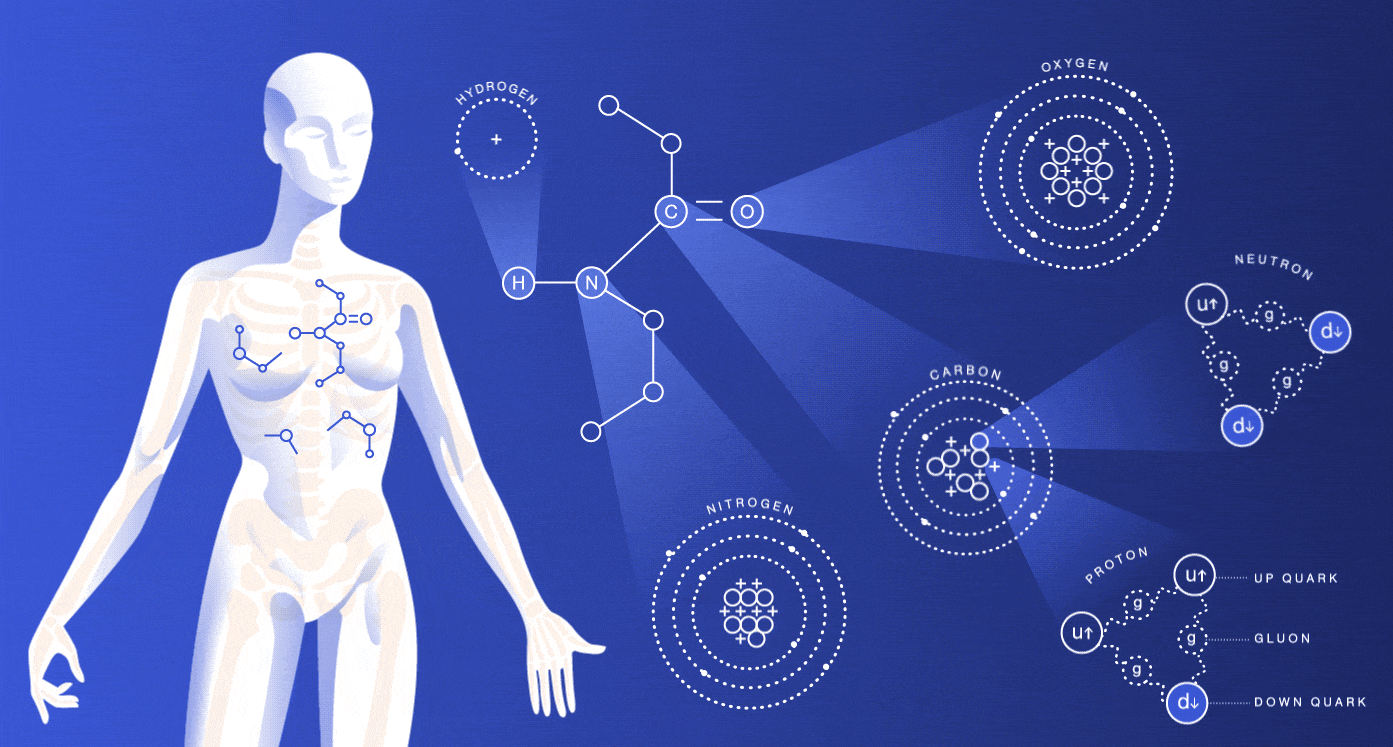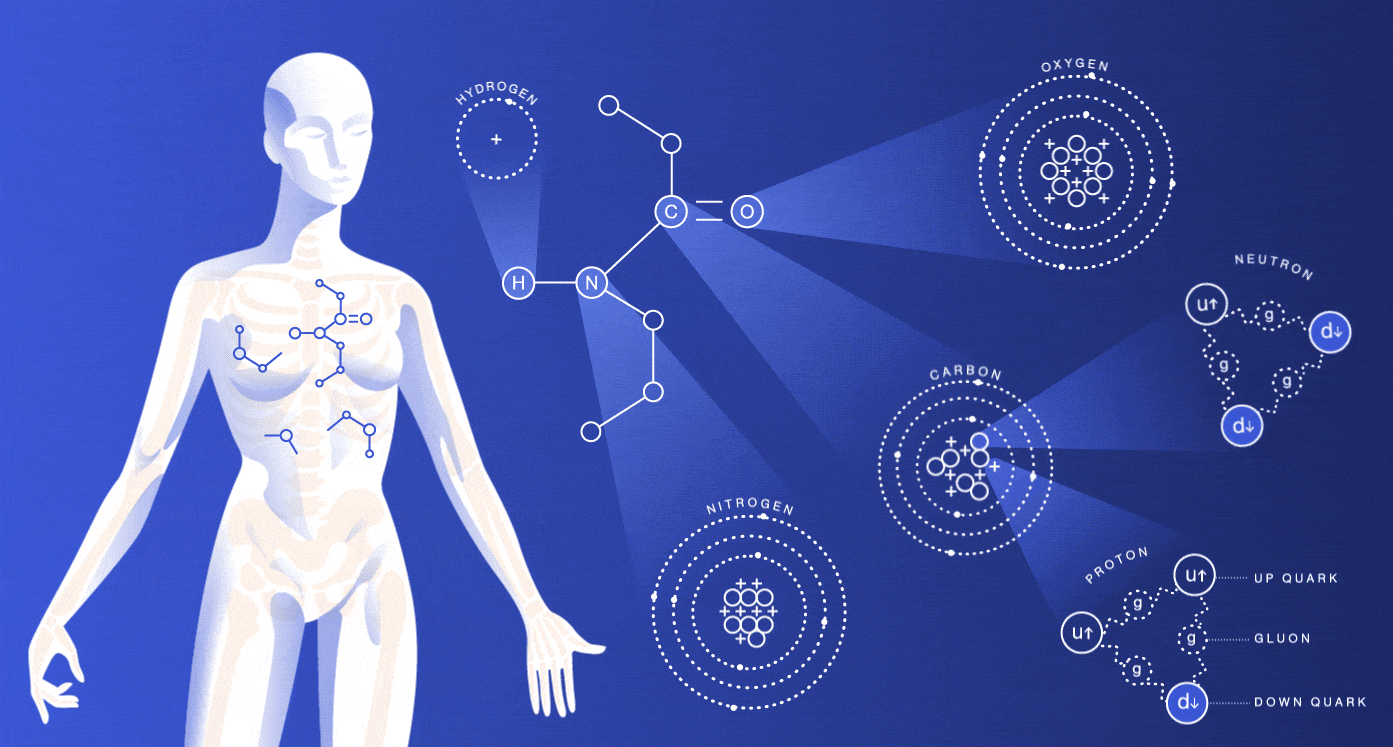
The particles we’re made of
About 99 percent of your body is made up of atoms of hydrogen, carbon, nitrogen and oxygen. You also contain much smaller amounts of the other elements that are essential for life.
While most of the cells in your body regenerate every seven to 15 years, many of the particles that make up those cells have actually existed for millions of millennia. The hydrogen atoms in you were produced in the big bang, and the carbon, nitrogen and oxygen atoms were made in burning stars. The very heavy elements in you were made in exploding stars.
The size of an atom is governed by the average location of its electrons. Nuclei are around 100,000 times smaller than the atoms they’re housed in. If the nucleus were the size of a peanut, the atom would be about the size of a baseball stadium. If we lost all the dead space inside our atoms, we would each be able to fit into a particle of lead dust, and the entire human race would fit into the volume of a sugar cube.
As you might guess, these spaced-out particles make up only a tiny portion of your mass. The protons and neutrons inside of an atom’s nucleus are each made up of three quarks. The mass of the quarks, which comes from their interaction with the Higgs field, accounts for just a few percent of the mass of a proton or neutron. Gluons, carriers of the strong nuclear force that holds these quarks together, are completely massless.
If your mass doesn’t come from the masses of these particles, where does it come from? Energy. Scientists believe that almost all of your body’s mass comes from the kinetic energy of the quarks and the binding energy of the gluons.
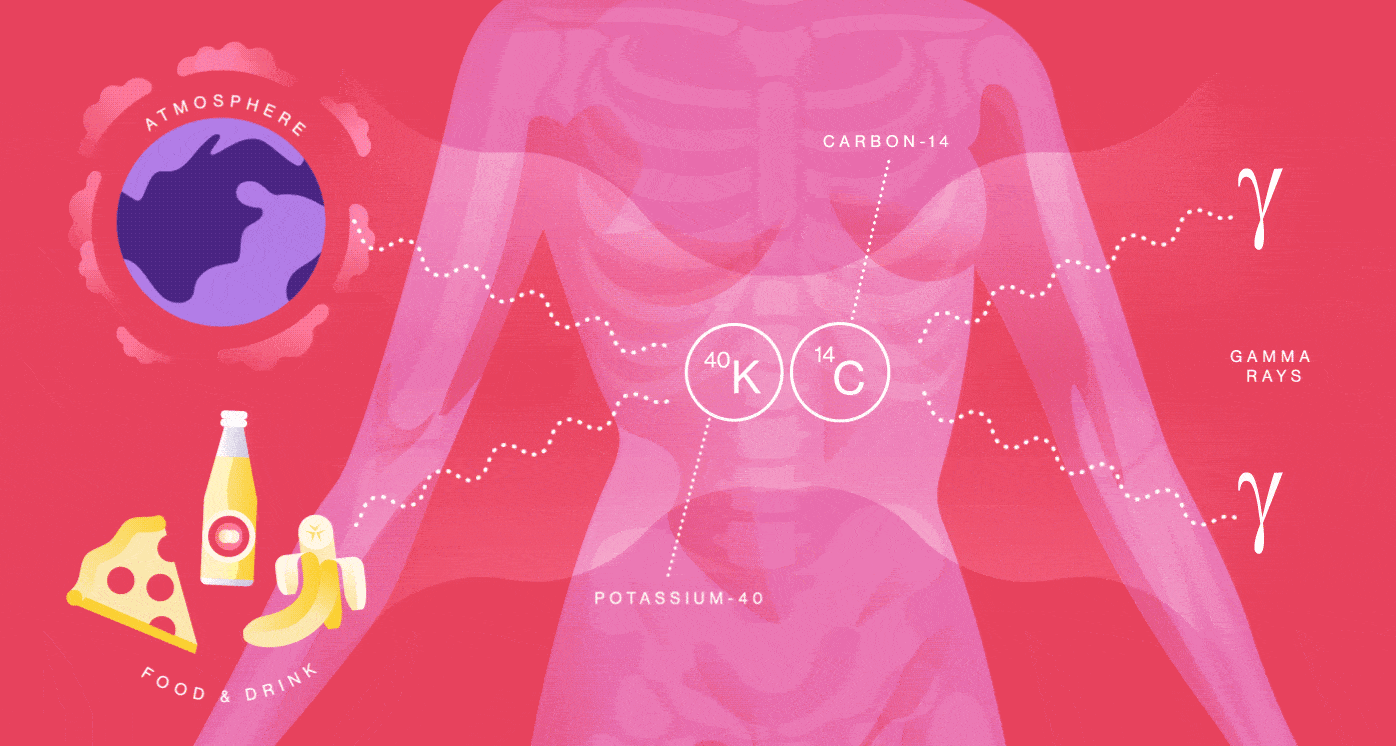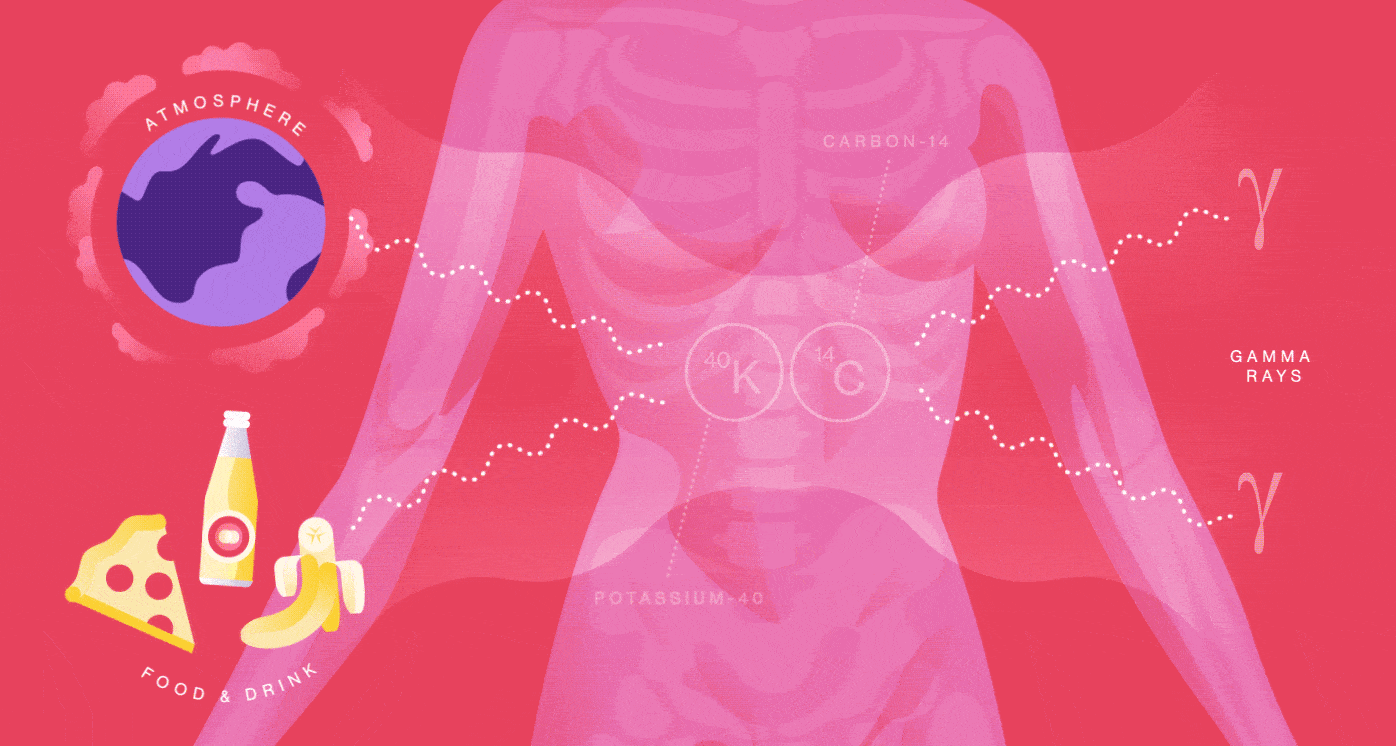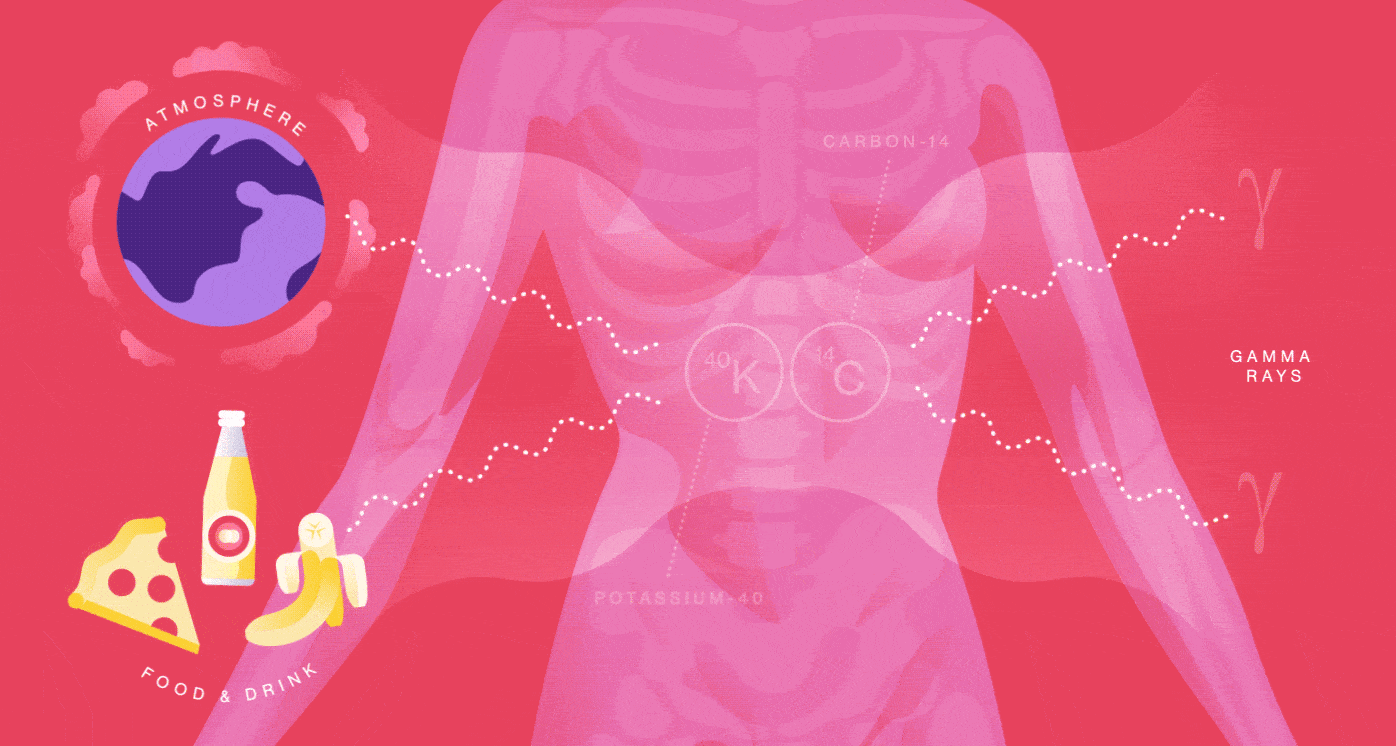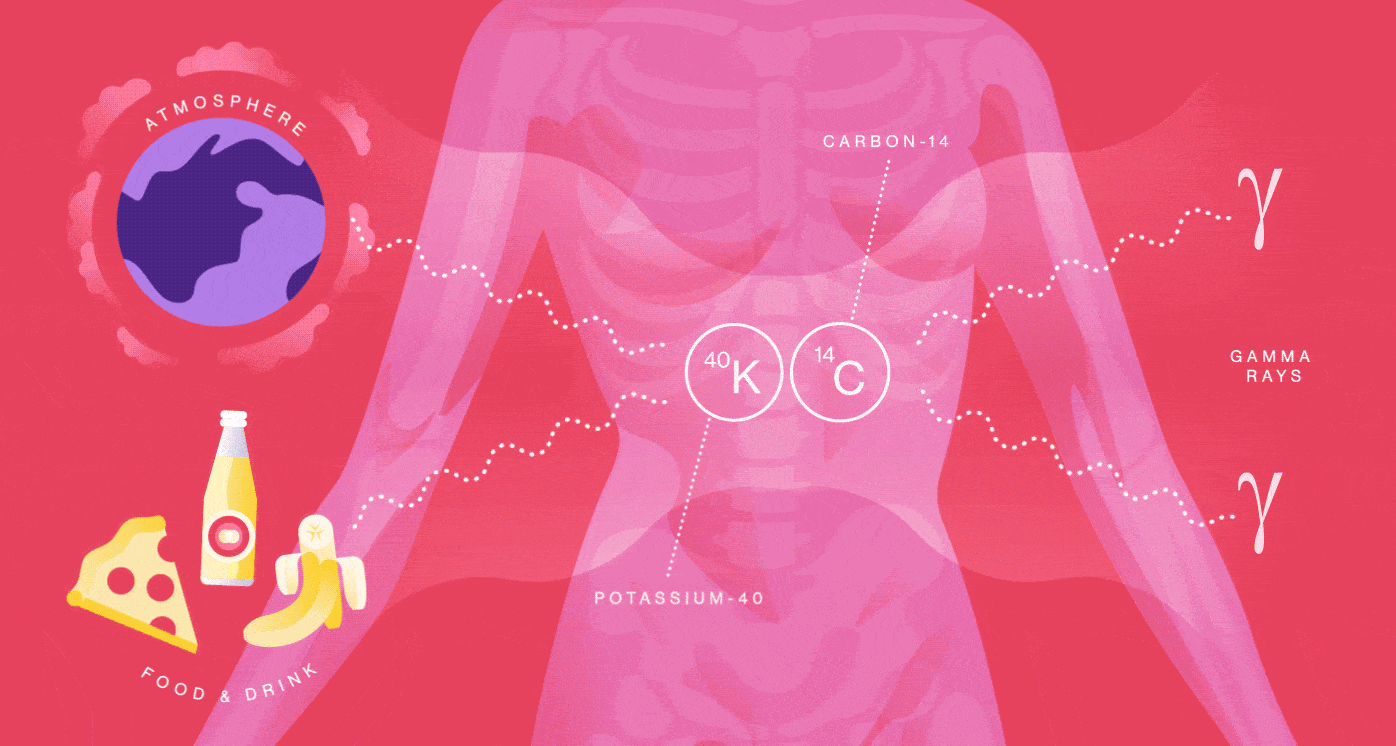
The particles we make
Your body is a small-scale mine of radioactive particles. You receive an annual 40-millirem dose from the natural radioactivity originating inside of you. That’s the same amount of radiation you’d be exposed to from having four chest X-rays. Your radiation dose level can go up by one or two millirem for every eight hours you spend sleeping next to your similarly radioactive loved one.
You emit radiation because many of the foods you eat, the beverages you drink and even the air you breathe contain radionuclides such as Potassium-40 and Carbon-14. They are incorporated into your molecules and eventually decay and produce radiation in your body.
When Potassium-40 decays, it releases a positron, the electron’s antimatter twin, so you also contain a small amount of antimatter. The average human produces more than 4000 positrons per day, about 180 per hour. But it’s not long before these positrons bump into your electrons and annihilate into radiation in the form of gamma rays.
The particles we meet
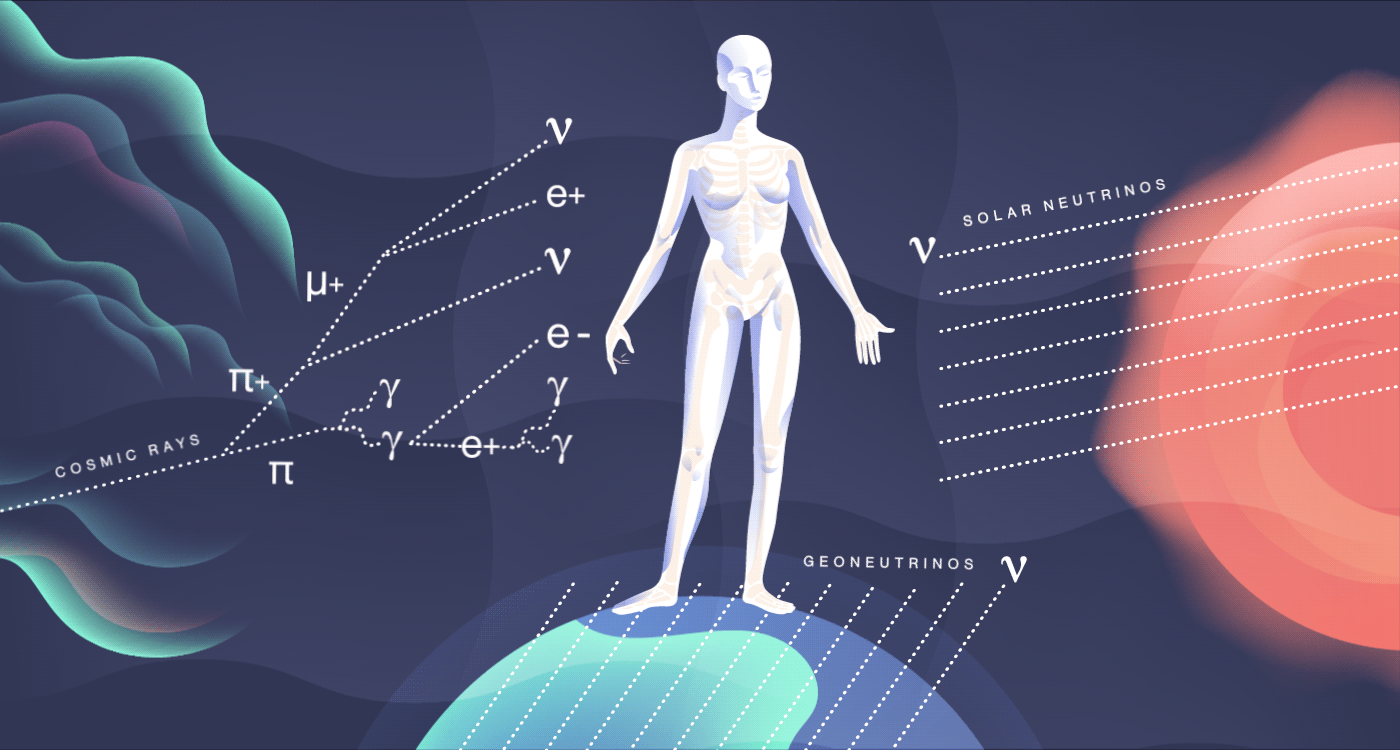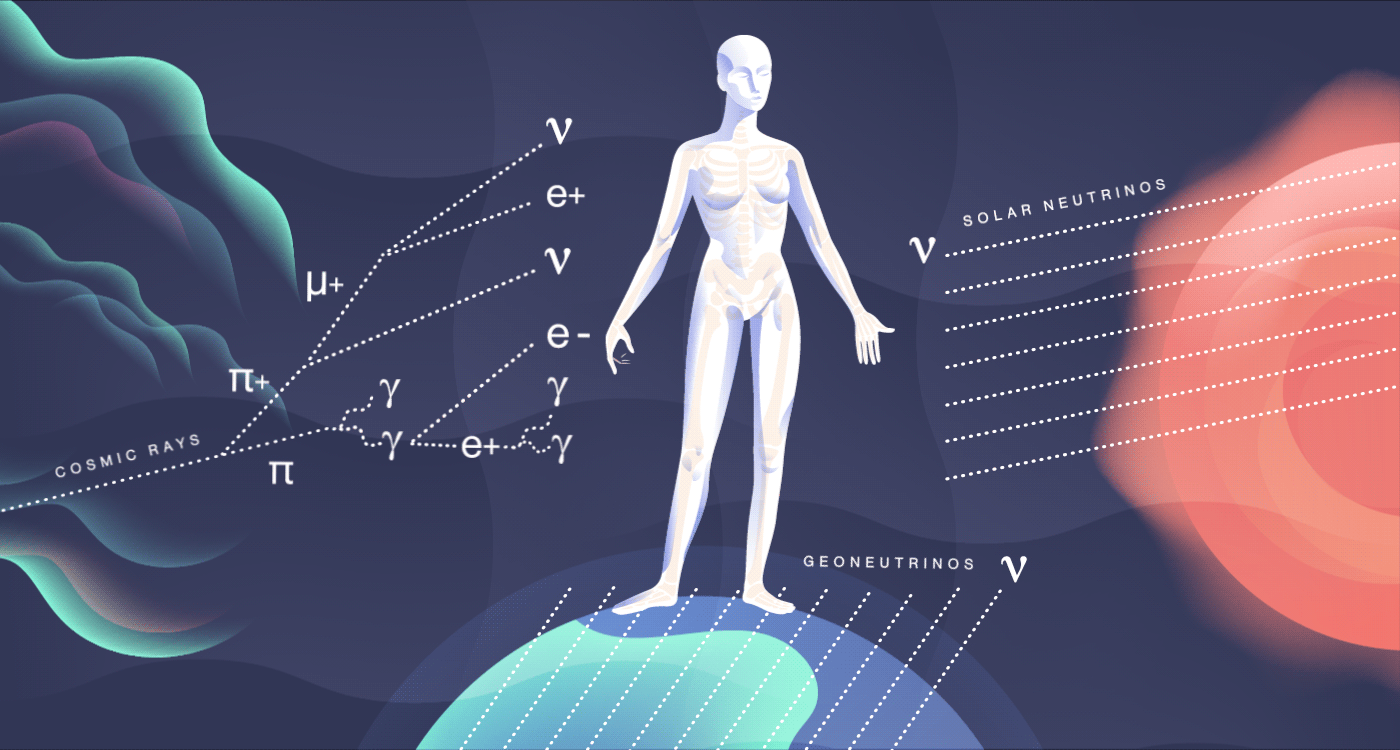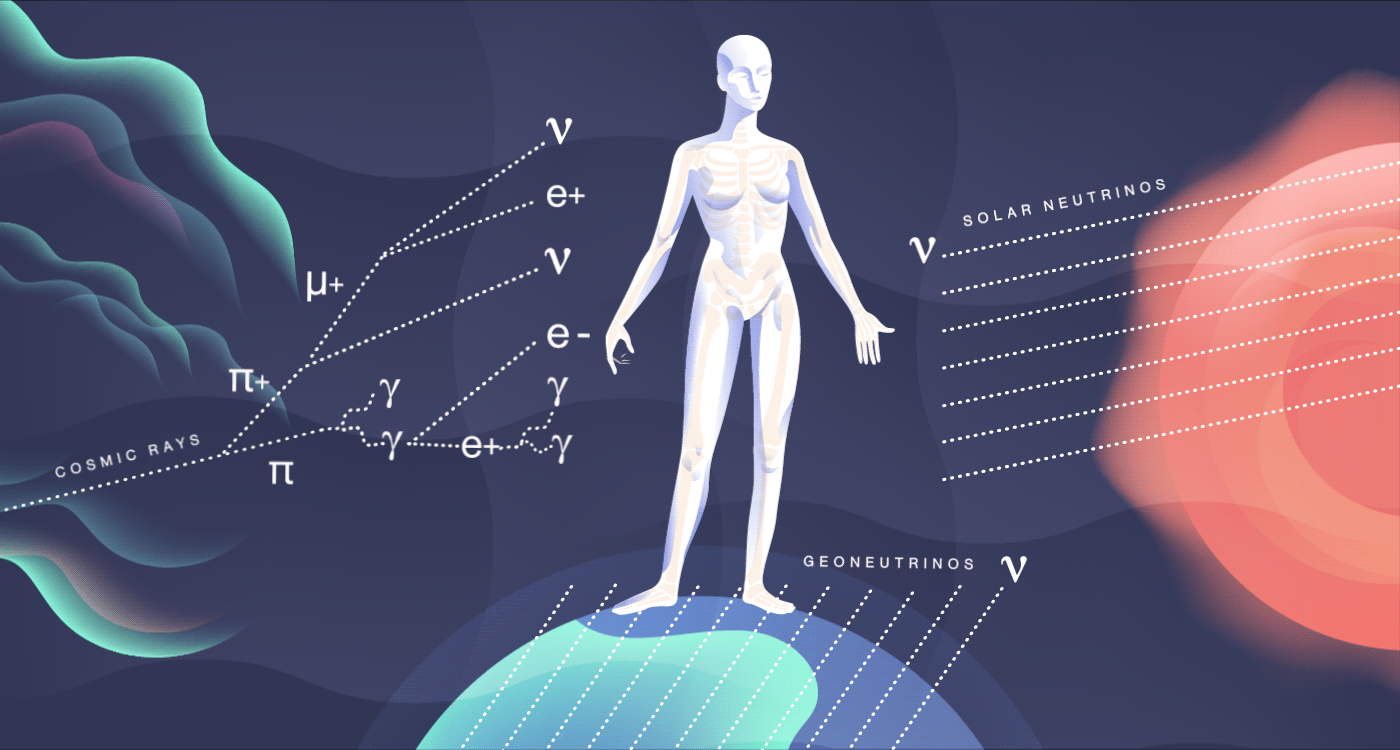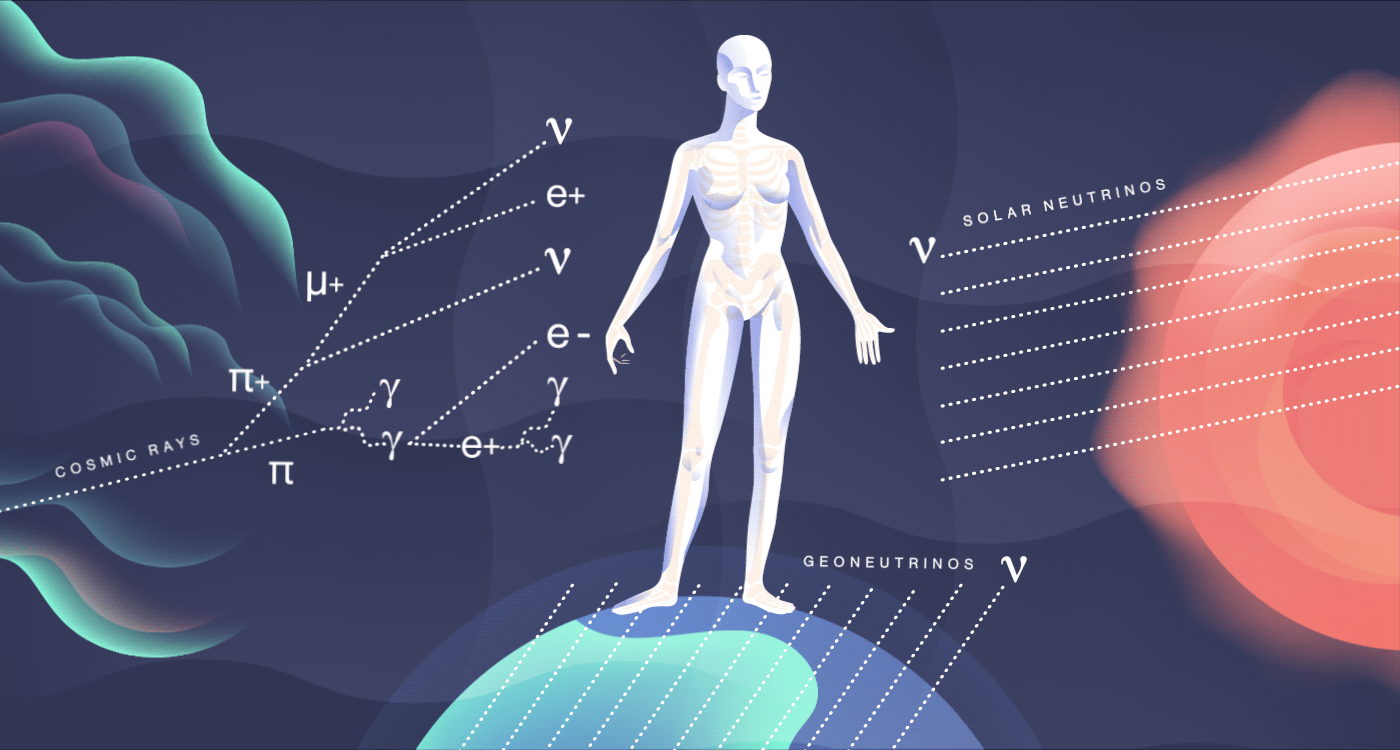
The radioactivity born inside your body is only a fraction of the radiation you naturally (and harmlessly) come in contact with on an everyday basis. The average American receives a radiation dose of about 620 millirem every year. The food you eat, the house you live in and the rocks and soil you walk on all expose you to low levels of radioactivity. Just eating a Brazil nut or going to the dentist can up your radiation dose level by a few millirem. Smoking cigarettes can increase it up to 16,000 millirem.
Cosmic rays, high-energy radiation from outer space, constantly smack into our atmosphere. There, they collide with other nuclei and produce mesons, many of which decay into particles such as muons and neutrinos. All of these shower down on the surface of the Earth and pass through you at a rate of about 10 per second. They add about 27 millirem to your yearly dose of radiation. These cosmic particles can sometimes disrupt our genetics, causing subtle mutations, and may be a contributing factor in evolution.
In addition to bombarding us with photons that dictate the way we see the world around us, our sun also releases an onslaught of particles called neutrinos. Neutrinos are constant visitors in your body, zipping through at a rate of nearly 100 trillion every second. Aside from the sun, neutrinos stream out from other sources, including nuclear reactions in other stars and on our own planet.
Many neutrinos have been around since the first few seconds of the early universe, outdating even your own atoms. But these particles are so weakly interacting that they pass right through you, leaving no sign of their visit.
You are also likely facing a constant shower of particles of dark matter. Dark matter doesn’t emit, reflect or absorb light, making it quite hard to detect, yet scientists think it makes up about 80 percent of the matter in the universe.
Looking at the density of dark matter throughout the universe, scientists calculate that hundreds of thousands of these particles might be passing through you every second, colliding with your atoms about once a minute. But dark matter doesn’t interact very strongly with the matter you’re made of, so they are unlikely to have any noticeable effects on your body.
The next time you’re wondering how particle physics applies to your life, just take a look inside yourself.